
Working together to develop better patient information for clinical trials: the MAPLE project
Vikki Wylde, Catherine Jameson, Emma Johnson and Kirsty Roberts
- Our work increasing diversity in clinical trials
- Health inequalities
- Lived experience
Clinical trials are important for making sure that medical treatments are safe and work well. For trials to benefit everyone they must include people from all backgrounds. However, the UK’s diverse population is not always reflected in the people who take part.
One major barrier to taking part in clinical trials are the over-complicated and non-inclusive patient information leaflets (PILs). We wanted to tackle this barrier by making the information easier to understand and answering the questions that really matter to people. By doing so, we hoped to help make trials more representative of all those living in the UK.
What is the MAPLE project?
MAPLE stands for Making trials more accessible through better patient information leaflets. It involves researchers from the University of Bristol working in partnership with National Voices, minoritised community organisations, charities and patients.
The aim: to co-design practical resources to help researchers create visual, inclusive, and accessible PILs for clinical trials.
Co-design in action
In MAPLE we wanted to work in equal partnership with communities – to respect the expertise of those with lived experience of the barrier we were addressing.
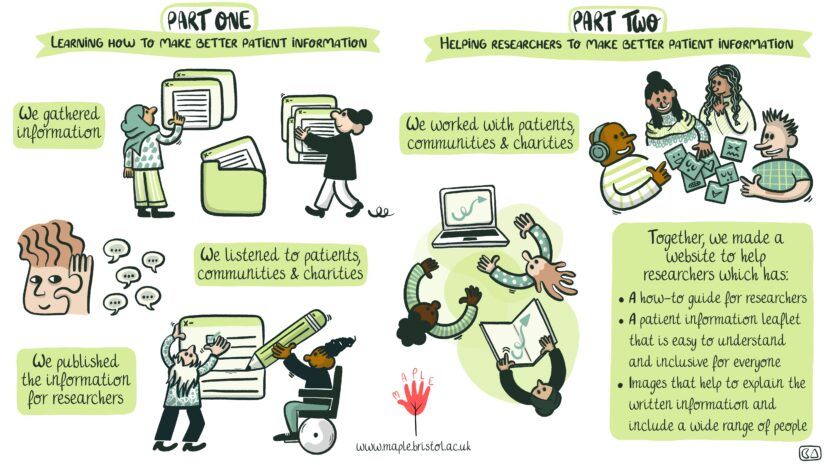
MAPLE Part 1
We met with two racially minoritised groups, two groups of adults with learning disabilities and/or autism and a patient advisory group. National Voices held an online workshop with representatives from 18 organisations and 5 lived experience partners. We listened to people’s experiences and views of health research. We asked what they liked and didn’t like about real-life examples of PILs and what they would like to see in them. We published information for researchers to share what we found.
MAPLE Part 2
Using insights from Part 1 we designed a new, prototype PIL and then worked together with people to make it better. We held 19 meetings with nine different groups. This included the original groups, and we worked with more communities, including Chinese and Eastern European groups. Key suggestions to improve the template included:
- Making the first page immediately relevant to the reader with general information later.
- Simplifying wording and giving transparent information around what a trial will involve, safety and how people’s information will be used.
- Including a ‘hello’ at the top of the leaflet in multiple languages to welcome people whose first language is not English.
We also asked about clear and culturally inclusive images to help explain the PIL sections. Visual information is not just for decoration; the right images can help understanding, bridge language barriers, show representation of diversity and help people from marginalised communities to feel included.
To ensure broad representation, National Voices brought together a group of charities and patient advocates experienced in public involvement. This meant we could also include views from the Roma community, LGBTQIA+ and Trans community support charities, Learning Disability England and people living with physical and mental health conditions. This group became our oversight group. They helped to make final decisions where community groups disagreed and provided expert help with accessibility and representation.
What we made
A website to help researchers create more inclusive and accessible PILs including:
- A modifiable PIL template
- A library of inclusive images
- Accessibility guidance
- Guidance on adapting the PIL for specific trials
Take home messages
Accessibility is a design responsibility. Low literacy isn’t the barrier to taking part in clinical trials – the barrier is overly complex, non-inclusive information. Researchers must take responsibility to change how they give trial information. By giving simple, clear and inclusive PILs, we help dismantle one barrier to more diverse clinical trials and create results that benefit all in our society.
If you have any questions or would like to discuss this template, you’re welcome to contact the authors:
emma.johnson@bristol.ac.uk catherine.jameson@bristol.ac.uk , kirsty.roberts@bristol.ac.uk and vikki.wylde@bristol.ac.uk