
Beyond compliance: Why patient engagement must shape medical device design
- Integrated care
- Person-centred care
Medical devices don’t exist in clinical isolation. They exist in someone’s daily life: in swimming pools, workplaces, bedrooms, and social settings. Yet too often, device manufacturers and prescribers sometimes treat the full patient experience as an afterthought rather than a design principle.
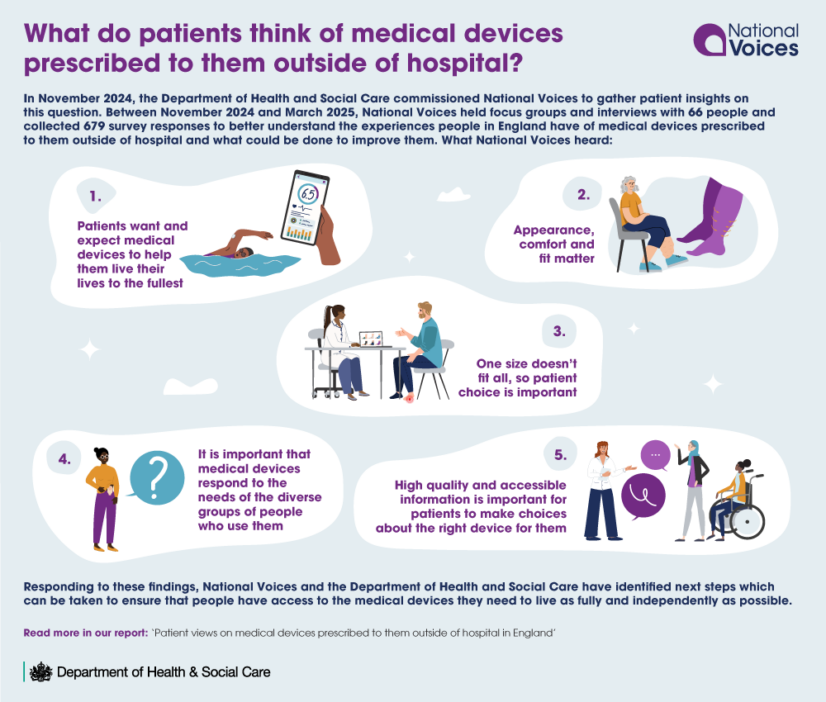
Our recent research with the Department of Health and Social Care gathered insights from 679 survey responses and 66 in-depth conversations with people who use medical devices prescribed under Part IX of the NHS Drug Tariff. The findings reveal a stark gap between what manufacturers prioritise and what patients actually need to live full lives. Read the full report here.
What we heard
Five themes emerged with striking consistency across different devices and user groups:
- Medical devices should enable life, not restrict it.
- Appearance, comfort and fit aren’t cosmetic concerns—they’re fundamental.
- One size rarely fits all.
- Design must respond to diversity.
- Information quality determines outcomes.
Why this matters beyond individual experience
Katie-Rose, a National Voices Lived Experience Partner who supported our research, articulated something crucial in her recent blog: “If you prescribe a product that actually fits with someone’s life, it has a real impact on their mental wellbeing.”
Better physical experience reduces depression, decreases medication needs, and improves overall outcomes. The cost-benefit calculation changes entirely when you factor in avoided mental health interventions and improved adherence.
She also reminded us: “Anyone can end up needing these devices.” This isn’t about ‘other people’—it’s about designing for the reality that any of us might need these products at any point in our lives.
This is just the beginning
This project engaged hundreds of people and generated clear evidence. But Katie-Rose voiced a critical concern: “If the recommendations aren’t acted upon, it would feel very disrespectful. It sends the message that people’s voices don’t really matter and risks becoming a tick-box exercise.”
Patient engagement cannot be extractive. We cannot ask people to share difficult experiences, then file the findings away. The recommendations must drive action and this project should catalyse ongoing engagement, not conclude it.
Practical steps for manufacturers
Based on our findings, manufacturers should:
- Establish ongoing patient advisory groups.
- Conduct accessibility testing as standard practice.
- Co-design with people at risk of experiencing inequalities.
- Research what matters beyond clinical function.
- Make ingredient and allergen information easily accessible.
Ongoing patient engagement
DHSC has committed to concrete next steps, including introducing a ‘value add’ component to the Quality Evaluation Framework that rewards manufacturers who address health inequalities. This creates market incentive for equity-focused design.
But real change requires manufacturers to recognise that patient engagement isn’t just a regulatory requirement, it’s also a source of insight that leads to better products, better outcomes, and ultimately better business.
Many of the people who completed our survey and shared their experiences in interviews and focus groups said how important and rewarding it felt to be listened to. Because as Katie-Rose said: “What I went through was never going to be easy, but being able to turn that experience into something constructive has been really meaningful.”
Read the full report here.
Please get in touch with Louis if you want to discuss further: louis.horsley@nationalvoices.org.uk